In Part I of this article, we talked about composition of our medicines, & the drug development process innovator companies follow to get their drug patented. In this article, we’ll talk about what happens once this patent expires & the exclusivity period ends.
The Competition Begins: Generics & Biosimilars
Once the patents or other periods of exclusivity expire, the branded drug becomes a generic drug. Now, other companies can file ANDAs (Abbreviated New Drug Application) to the FDA for approval to sell the generic versions of this drug.
ANDA (Abbreviated New Drug Application)
Abbreviated, because the approval process is much shorter than a NDA, as generic drug companies don’t need to conduct clinical trial for the drug.
Instead, they must scientifically demonstrate that their product is bioequivalence (measured by comparing the rate and extent of absorption of the generic drug to that of the innovator drug in healthy volunteers).
Therefore, a generic drug company’s R&D is limited to reverse engineer the innovator drug to prove Bioequivalence to the original drug.
A generic can take 2-4yrs to develop & costs USD $1-$2 millions
First-To-File Wins
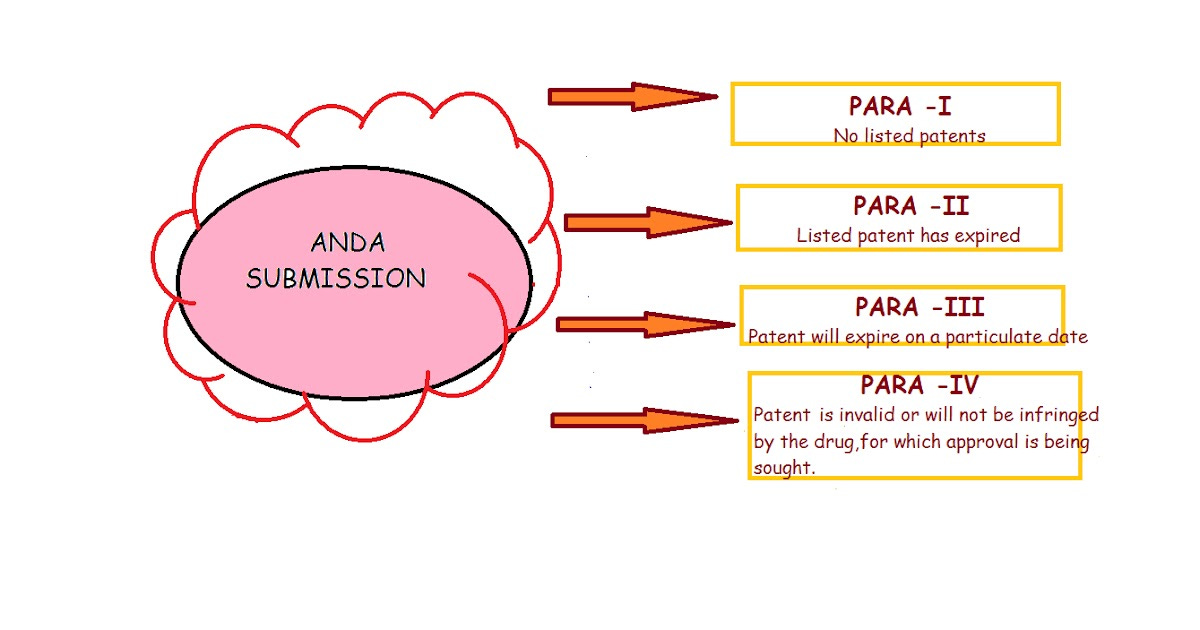
Companies file ANDAs under one of the four patent certifications, also known as paragraphs.
Para I
This certification states that there are no listed patents for the generic drug. In this case, FDA usually approves the drug immediately. These drugs face the highest competition & are basically commodities.
Para II
This certification states that the listed patent for the drug has expired. In this case as well, FDA may approve the drug immediately. These drugs face high competition too.
Para III
This certification states that the patent on a listed drug has not expired yet, but will expire on a particular date. In this case, the FDA may approve the drug with effect from the day of patent expiry.
Para IV
This certification is sought after the most. In a Para IV filing, a generic company challenges the patent of the innovator, stating that its invalid & shouldn’t have been granted in the first place or, that the generic doesn’t infringe on the patent.
The first generic company to challenge the patent & win in court, is granted First-To-File (FTF) status i.e. 180 days of exclusivity to market the drug. No competitor can enter the market for 6 months — this is an incentive given by the FDA to generic companies for taking down fraudulent patents & thus, greatly reducing the price of the drug.
Generic Drugs: When patent expires for a Chemical Drug
These are identical--or bioequivalent--to a branded chemical drug in dosage form, safety, strength, route of administration, quality, performance characteristics and intended use.
Although generic drugs are chemically identical to their branded counterparts, they are typically sold at substantial discounts from the branded price.
There are 4 types of generic drugs:
1. Authorized Generics
The innovator company doesn’t discontinue a product, just because its patent has expired. Instead, they lower the price of the drug & sell it as authorized generic.
These are often sold for a slight premium over branded generics.
2. Branded Generics
Though these are bioequivalent to the original drug, they may vary in shape, colour & flavour. Companies sell these drugs under their own brand name & therefore incur branding & marketing costs.
Branded generics are sold for a slight premium for over unbranded generics.
They are sold mostly to the public, as they prefer branded products for assurance of quality & are willing to pay a small premium for it.
3. Unbranded Generics
These are also known as commodity generics, & are sold just by the name of the API in the drug. They are not differentiated product as they have no branding, therefore they save on marketing.
These are mostly bought by the govt. or institutions who provide highly subsidized or free medication to the masses & don’t want to pay premium for the brand.
Out of all 4 types, unbranded generic space has the most players, i.e. most competition.
4. Specialty Generics
These are generic versions of specialty drugs.
Specialty Drugs: High value drugs that are used to treat chronic & complex diseases. Sometimes, these drugs also have complex delivery systems. These are very expensive drugs.
Specialty generics usually sell at a higher price than all other generics.
Biosimilars: When patent expires for a Biologic
A biosimilar is highly similar to and has no clinically meaningful differences from an existing FDA-approved biologic, called a reference product.
Similar to ANDAs in the case of generics, when the patent for a biologic expires, companies file ABLA (Abbreviated Biologics License Application) to seek approval to manufacture biosimilars of it.
Compared with a reference product, biosimilars:
Are made with the same types of living sources
Are given to the patient in the same way
Have the same strength, dosage, potential treatment benefits, and potential side effects .
A proposed biosimilar is compared to and evaluated against the reference product to verify that it is highly similar to and has no clinically meaningful differences in terms of safety, purity, and potency (i.e., safety and effectiveness) from the reference product.
Note: They should be highly similar in duplication, (& not identical like in the case of generics) to the reference product to accomplish therapeutic & clinical result.
This is because a biologic usually contain a mix of many slight variations of a protein. This mix can never be exactly the same in each dose or batch of the product.
Biosimilars can take 8-10yrs to develop & cost USD $100-$200 million.
Hope you enjoyed the read & understood the pharma industry a little better. In upcoming articles we’ll delve deeper into the pharma industry.
If you found this article helpful, your friends might too. Feel free to share 😊.